1. introduction & history
Isoprene rubber (IR) is a synthetically produced elastomer material that is almost identical to natural rubber (NR) in terms of its molecular structure. IR occupies a special position within the rubber family, as it combines the positive properties of natural rubber - such as high elasticity and good tear resistance - with the purity and reproducibility of industrial synthesis.
The historical development of IR began in the middle of the 20th century, when the increasing demand for consistent quality in technical applications revealed the limits of the natural raw material. Natural rubber, obtained from the latex of the rubber tree Hevea brasiliensis, isoprene is subject to fluctuations in composition and quality, which are influenced by cultivation and harvesting conditions. The introduction of modern polymerisation processes has made it possible to extract isoprene from petrochemical sources and polymerise it into high-purity polyisoprene.
The raw material basis for IR is the monomer isoprene (2-methyl-1,3-butadiene). It is separated industrially from the C₅ fraction, which is produced during the pyrolysis of naphtha.
Production on an industrial scale is carried out by means of solution polymerisation, often using Ziegler-Natta catalysts, with the aim of achieving the highest possible cis-1,4-isoprene content in the polymer chains. This controlled production allows precise control of the physical properties, which makes IR particularly suitable for applications with high purity and quality requirements.
2. chemical composition of isoprene rubber
Isoprene, the basic monomer of IR, is chemically known as 2-methyl-1,3-butadiene.
The two double bonds of the monomer enable targeted polymerisation by means of solution polymerisation in the presence of Ziegler-Natta catalysts. The result is polyisoprene with a high content of cis-1,4-isoprene (> 98 %). This corresponds to the polymer structure of natural rubber, which means that the physical properties are very similar.
The structural formula of IR shows a linear polymer chain in which the isoprene units are connected to each other via 1,4-links. Compared to natural rubber, IR has a narrower molecular weight distribution and fewer impurities such as proteins, lipids or resins. This purity is crucial for applications in which biological by-products are undesirable, e.g. those that can trigger allergies. Compared to NR, this property makes IR the predestined rubber for use in medical technology or food contact applications, for example.
After polymerisation, the raw material is usually available as whitish to slightly yellowish granules or in bale form and is highly soluble in non-polar organic solvents such as benzene or toluene.
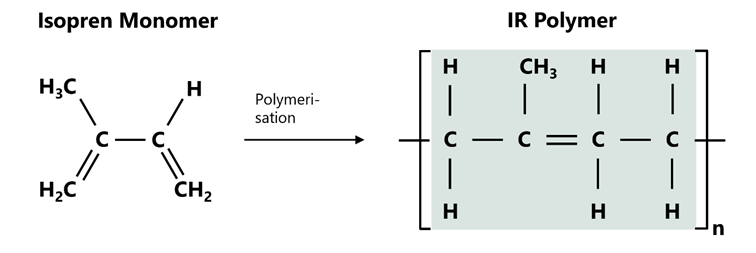
Figure 1: Polymer structure of IR.
Vulcanisation mechanisms
Isoprene rubber is an unsaturated elastomer whose double bonds in the main chain enable a variety of cross-linking reactions. The most commonly used method is sulphur vulcanisation. The double bonds of the polymer chain react with sulphur atoms mediated by accelerators (e.g. thiazoles, sulfenamides) and activators (e.g. zinc oxide, stearic acid). Accelerators and activators have a catalytic effect and are not incorporated into the chains or cross-linking bridges. The formation of intermediate complexes with the polymer chains and the sulphur atoms accelerates the cross-linking reaction or reduces its activation energy. Sulphur bridges are formed between the macromolecules, the length of which (mono-, di- or polysulphide bridges) significantly influences the properties of the end product. Short bridges increase the heat resistance and hardness, longer bridges improve the dynamic load-bearing capacity and tear resistance, but at the same time increase the compression set, as long sulphur chains can migrate along the polymer chains (self-healing).
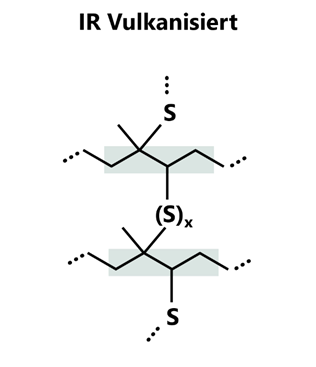
Figure 2: Schematic representation of cross-linking by sulphur bridges of IR.
Alternatively, peroxide crosslinking can be used, especially if thermal stability and media resistance are the main focus. This creates direct C-C bridges between the polymer chains, which are more temperature-stable and oxidation-resistant than sulphur bridges, but usually lead to lower elasticity and poorer dynamic properties.
Other possible processes include resin crosslinking (especially with phenolic resins) for special hardness and swelling resistance properties as well as radiation crosslinking using electron or γ-irradiation in high-purity applications. The choice of vulcanisation system is always adapted to the subsequent operating conditions, standard requirements and production processes and is also based on economic considerations.
3. properties & special features of isoprene rubber
IR (isoprene rubber) is characterised by a property profile that largely corresponds to that of natural rubber (NR), but has a significantly higher reproducibility due to its controlled synthesis. Its outstanding features are high elasticity, excellent resilience and good low-temperature flexibility down to -60 °C, which makes it ideal for dynamically stressed components.
In the unstretched state, the chains of the IR are highly entangled, making the elastomer very elastic. Under tensile stress, the chains can partially align parallel to each other and enter into Van der Waals interactions. These partially crystalline areas act like physical cross-linking points and increase the tensile strength and tear resistance. This so-called strain crystallisation is reversible, so that the unloaded material retains its elasticity.
IR typically has a tensile strength of 20-25 MPa (unreinforced) and an elongation at break of over 500 %. The targeted addition of fillers (e.g. carbon black, silica) allows abrasion resistance, hardness and rigidity to be customised to the requirements of the application. The material exhibits excellent damping behaviour at low temperatures and remains functional over a wide temperature range.
Like NR, isoprene rubber is sensitive to oxygen, ozone and UV radiation, as these attack the double bonds in the polymer chain and thus cause chain breaks. Anti-ageing agents (antioxidants, antiozonants) are therefore required for protection. In terms of media resistance, IR as a non-polar polymer shows good resistance to water and diluted acids, but only limited resistance to oils, hydrocarbons and organic solvents.
- Advantages over NR: Higher purity, lower protein content (no allergenic proteins), consistent quality regardless of cultivation and harvesting conditions, specifically adjustable molecular weights.
- Disadvantages compared to NR: Similar susceptibility to thermal and oxidative ageing, comparable restrictions in terms of oil and fuel resistance.
- Compared to SBR (styrene-butadiene rubber): Better low-temperature flexibility and dynamic properties, but lower heat ageing resistance.
| Nature Rubber | Styrene-butadiene Rubber | Ethylene- Propylene diene Rubber | Butyl rubber | Chloroprene Rubber | Nitrile Rubber | Hydrogenated nitrile Rubber | Flour- Rubber | Silicone Rubber | Fluorosilicone | ||
| International abbreviation | NR | SBR | EPDM | IIR | CR | NBR | HNBR | FKM | VMQ | FVMQ | |
| Hardness range (in Shore) | 25A-70D | 20A-95A | 20A-95A | 30A-80A | 20A-90A | 20A-75D | 50A-95A | 50A-90A | 20A-90A | 40A-80A | |
| Mechanical Properties for Room temp. | Tear resistance | 8 | 6 | 5 | 4 | 6 | 6 | 8 | 4 | 3 | 3 |
| Elongation at break | 8 | 6 | 5 | 8 | 6 | 6 | 5 | 4 | 8 | 4 | |
| Rebound resilience | 8 | 6 | 6 | 0 | 4 | 4 | 4 | 0 | 6 | 2 | |
| Tear propagation resistance | 8 | 4 | 4 | 4 | 6 | 4 | 3 | 2 | 2 | 2 | |
| Abrasion resistance | 5 | 6 | 5 | 3 | 6 | 7 | 8 | 3 | 1 | 1 | |
| Compression set | at max. continuous operating temperature | 4 | 4 | 2 | 4 | 3 | 3 | 4 | 0 | 0 | 0 |
| at room temperature | 2 | 3 | 0 | 3 | 3 | 2 | 4 | 1 | 0 | 0 | |
| Thermal behaviour | Cooling behaviour (Tg) up to °C | -55 | -45 | -50 | -60 | -40 | -45 | -40 | -30 | -50 | -65 |
| Max. Continuous operating temperature up to °C | 80 | 90 | 130 | 130 | 100 | 110 | 150 | 220 | 210 | 200 | |
| Resistance to | Petrol | 0 | 0 | 0 | 0 | 2 | 5 | 4 | 8 | 1 | 7 |
| Mineral oil (at 100 °C) | 0 | 0 | 0 | 0 | 4 | 8 | 8 | 8 | 4 | 7 | |
| Acids (aqueous inorganic acids at RT) | 2 | 2 | 8 | 8 | 5 | 3 | 4 | 8 | 4 | 4 | |
| Alkalis (aqueous inorganic alkalis at RT) | 4 | 3 | 8 | 8 | 5 | 2 | 4 | 8 | 2 | 2 | |
| Water (at 100 °C, distilled) | 3 | 3 | 8 | 8 | 4 | 4 | 6 | 8 | 5 | 4 | |
| Weather and ozone | 3 | 3 | 8 | 6 | 8 | 2 | 8 | 8 | 8 | 8 | |
Table 1: Properties of selected materials: 0 = unsuitable, 8 = very suitable
4 Areas of application for isoprene rubber
Isoprene rubber is the preferred choice when the positive properties of NR are required but a consistent and high-purity quality is needed - for example in medical technology products, high-precision seals or moulded parts subject to vibration.
IR is used in a wide range of technical and specialised applications where high elasticity, good mechanical properties and reproducible material quality are required. Due to its close structural relationship to natural rubber, it can be used as an equivalent substitute in many cases, but offers the advantage of consistent, batch-independent quality.
Dynamically stressed moulded parts:
IR is often used for components that work under repeated deformation and alternating loads, such as vibration dampers, membranes or flexible coupling elements. The high resilience gives these components a long service life even under cyclical stress.
Elastic sealing and waterproofing elements:
In applications where a soft, adaptable material is required for sealing, IR offers a good balance between elasticity and dimensional stability. Examples include O-rings, flange gaskets or sealing sleeves that are used in aqueous media or at low temperatures.
Medical and hygiene products:
Due to its purity and the absence of allergenic proteins, isoprene rubber is used in the manufacture of medical gloves, catheters and stoppers for pharmaceutical packaging. Its good tear resistance and elasticity ensure safety and comfort in single and multiple use applications.
Rubber threads and textile applications:
The combination of high elasticity, stretchability and resilience makes isoprene rubber a favoured material for rubber threads that are integrated into textiles, sporting goods or technical fabrics.
Special applications:
In combination with fillers and plasticisers, IR can be tailored to specific requirements, for example for conveyor belts with high flexibility, rubber rollers for printing and paper machines or airtight components for pneumatic systems.
The versatility of IR is based not only on its basic mechanical properties, but above all on the ability to control these precisely via formulation and vulcanisation, so that a wide range of customised products can be created.
