HNBR - high temperature and media resistance for demanding sealing applications
1. definition HNBR
HNBR elastomer is a synthetic rubber that is produced by hydrogenating NBR (hydrogenated nitrile butadiene rubber) and is available as colourless, light-coloured granules. HNBR offers a higher heat resistance of up to +150 °C and a higher ageing resistance than normal NBR with comparable resistance to wear, oils and fuels. HNBR is often used in applications that require mechanical strength in combination with chemically aggressive environments and high temperatures, such as timing belts, seals, plugs and bearing elements in railway technology, drive technology, mechanical engineering and the automotive sector.
History of HNBR
HNBR was developed in the late 1970s and early 1980s as a further development of its predecessor NBR, when the limits in terms of heat ageing and ozone resistance in automotive and industrial applications became apparent. HNBR was finally produced commercially for the first time in 1984 and, thanks to its combination of good chemical, physical and mechanical properties, has since been used in a wide range of applications in almost all branches of industry
Chemical composition of HNBR
Like its base material NBR, HNBR is a so-called copolymer. Copolymers consist of at least two different monomers that are repeated in linear polymer chains. In the case of HNBR, these linear chains consist of the σ-linked monomers acrylonitrile and 1,3-butadiene. The nitrile groups (-C≡N) characteristic of HNBR are attached to these polymer chains, which are responsible for some of the special properties of HNBR such as resistance to oils, greases and chemicals and make HNBR particularly interesting for applications in chemically demanding environments.
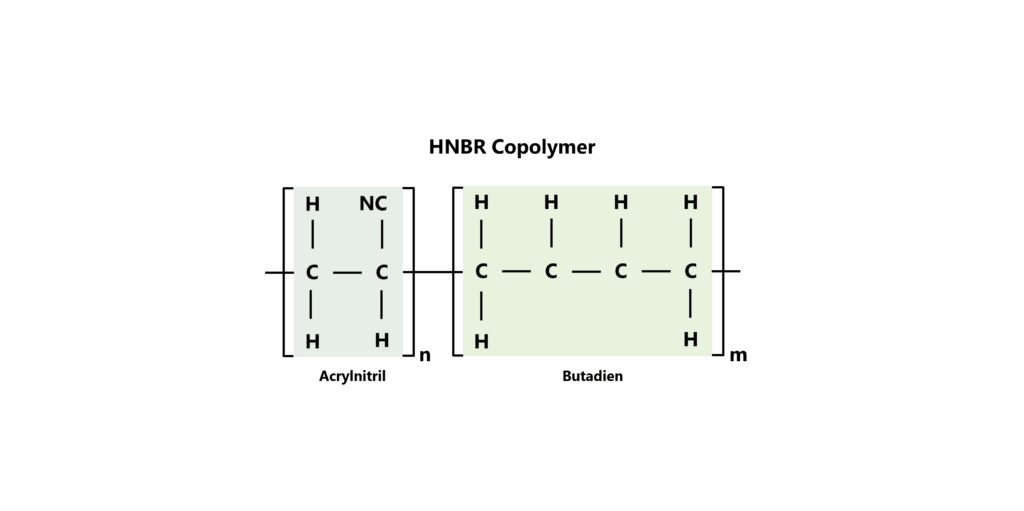
Figure 1: Illustration of an HNBR copolymer consisting of an acrylonitrile (left) and a butadiene (right)
Compared to the precursor polymer NBR, HNBR is characterised by the hydrogenation of the double bonds contained in NBR, whereby C=C double bonds become C-C single bonds. The degree of this saturation of double bonds can be varied, allowing the properties of the material to be specifically controlled. By increasing the saturation of the polymer chains, resistance to oxygen, heat and ozone can be significantly improved and the desired combination of low-temperature flexibility and media resistance can be realised.
If HNBR is not fully hydrogenated, the chain structure can also be chemically described as a terpolymer: In addition to the acrylonitrile units, both hydrogenated (saturated C-C segments) and unhydrogenated butadiene units (with C=C double bonds) are then present in the same polymer matrix. These residual double bonds act as defined reaction sites in certain crosslinking systems and enable a finer balance between dynamic properties, elasticity and resistance. The lower the residual unsaturation, the more heat, ozone and oxidation resistance typically come to the fore, while higher proportions of residual double bonds increase the crosslinking reactivity, but can limit the ageing stability to a greater extent.
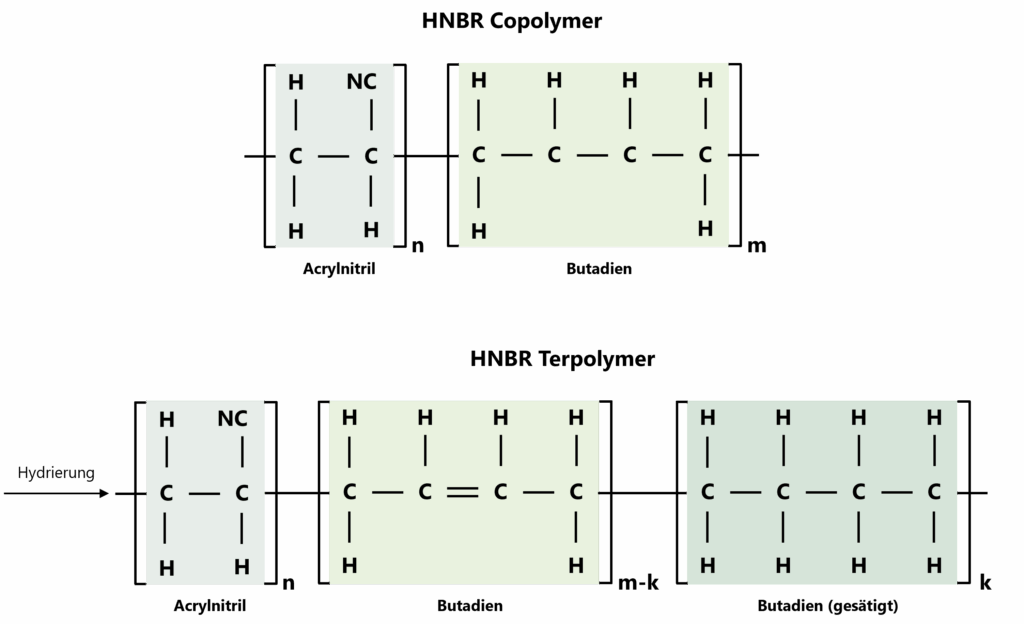
Figure 2: Hydrogenation of the C=C double bonds contained in the polymer (left) turns the HNBR copolymer into a more saturated HNBR terpolymer with predominantly C-C single bonds and remaining residual double bonds as reactive crosslinking sites (right).
2. HNBR properties & special features
HNBR combines a high tolerance to chemical and mechanical stresses with a high long-term temperature variability of -30 °C to 150 °C. The chemical and mechanical properties of HNBR are comparable to those of NBR. However, HNBR is characterised in particular by its significantly greater temperature variability compared to the industrial alternative NBR.
Physical properties of HNBR
The physical properties, in particular the long-term temperature resistance, are primarily due to the special vulcanisation process with organic peroxides. Due to the radical cross-linking, no foreign atoms remain in the material and closer cross-linking of the polymer chains is possible, making the material more resistant to thermal stress.
Chemical properties of HNBR
The chemical properties of HNBR are particularly evident in its high resistance to various chemicals and its durability under adverse weather conditions and ozone. HNBR is stable to water and water vapour up to a continuous temperature of 150 °C as well as to weak acids and bases and salt solutions at low temperatures. HNBR is also highly resistant to organic solvents such as aliphatic hydrocarbons, glycol-based coolants, heating oil and diesel as well as flame-retardant pressurised fluids. Due to the incorporated nitrile groups (-C≡N), HNBR materials are highly resistant to fats and oils of animal and vegetable origin. This extensive resistance to their environment makes HNBR materials extremely suitable for applications in chemically demanding environments. Furthermore, HNBR-based components are also suitable for use in drinking water systems. For this purpose, the rubber compound is subjected to special physical and microbiological tests and can therefore be used in compliance with FDA and KTW regulations.
Mechanical properties of HNBR
HNBR has a high tensile strength and is also abrasion-resistant. This enables durable seals in mechanically demanding positions. In addition, the flexibility of HNBR components can be specifically designed by varying the monomer content. A lower acrylonitrile content can increase the flexibility of the material, especially at low temperatures. In addition to its mechanical behaviour, HNBR also impresses with its particularly low permeability to gases and vapours. Thanks to its high resistance to ageing, materials made from HNBR retain this property over a long period of time.
3. processing of HNBR
Chemical production of HNBR
HNBR rubber is produced in two steps. First, the starting polymer NBR is produced from the two monomers acrylonitrile and 1,3-butadiene by emulsion polymerisation in water. The molar proportions of the monomers can be deliberately varied depending on the reaction control, which enables the properties of the subsequent materials to be specifically controlled.
In the second step, the precursor polymer NBR is further processed into the target polymer HNBR by catalytic hydrogenation. NBR with an acrylic content of 33-49 % is typically used for this. During catalytic hydrogenation, the double bonds between two carbon atoms in the NBR are saturated by hydrogen atoms, turning the double bond into a single bond. This means that all possible coordination sites of the carbon atoms contained are saturated with hydrogen atoms. Fully hydrogenated HNBR is therefore also referred to as saturated compounds or saturated HNBR. However, depending on the application and the desired property profile of the material, not all of the available double bonds are hydrogenated, but only some of them. In this case, we speak of partially hydrogenated or not fully saturated HNBR.
After the hydrogenation of NBR, HNBR is usually available as colourless, light-coloured granules and has good resistance to non-polar solvents.
Vulcanisation process of HNBR
The vulcanisation of polymers refers to the formation of a three-dimensional network between the individual linear polymer chains. This vulcanisation ultimately gives the materials their final properties. In the case of HNBR, sulphur or organic peroxides are used for this, whereby the vulcanisation techniques depend on the degree of hydrogenation of the HNBR.
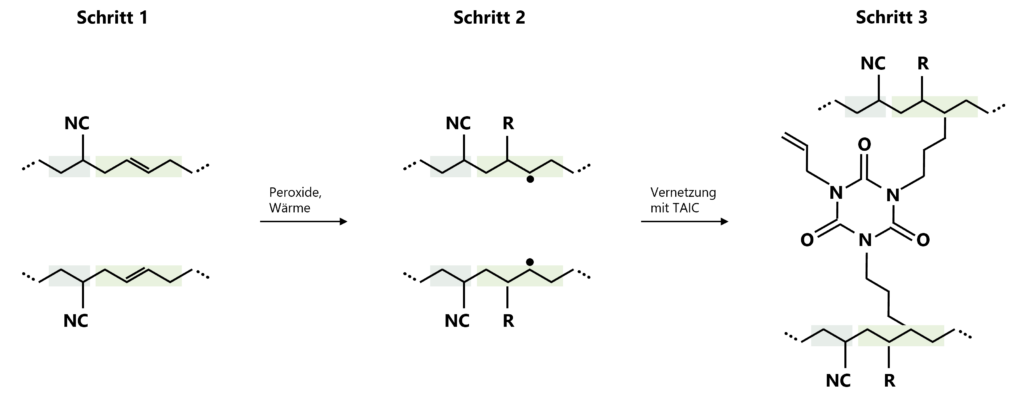
Figure 3: Vulcanisation of HNBR by peroxide radical formation and activation of the polymer chains (left) and subsequent crosslinking using a co-agent such as triallyl isocyanurate (TAIC) to increase the crosslinking density (right).
Partially hydrogenated HNBR can be vulcanised by means of so-called sulphur vulcanisation. In sulphur polymerisation, the sulphur atoms react with the double bonds of the polymer chains and coordinate between two double bonds of different polymer chains. A sulphur chain can connect two individual polymer chains and thus form a three-dimensional network across all polymer chains.
Saturated HNBR no longer has any double bonds, which is why sulphur is unsuitable as a vulcanisation reagent. Instead, organic peroxides are used, which do not remain in the final material. Organic peroxides decompose into two radicals at temperatures between 150 and 200 °C. These radicals attack the polymer chains, resulting in the formation of polymer chain radicals. When two of these polymer chain radicals meet, they recombine and form a chemical bond between the polymer chains. The newly formed chemical bond now represents the cross-linking between the polymer chains. By adding radical termination reagents, this vulcanisation process can be specifically stopped and the degree of cross-linking itself can be determined. This makes it possible to control the properties of the material through structural chemical design.
Industrial production
For the industrial processing of HNBR, the base polymer is mixed with various additives, fillers, vulcanising agents, accelerators, etc. in a rubber mixing plant. The starting chemicals are first kneaded and then processed either by extrusion into rubber strands or by calendering into mat material. The finished HNBR compound is then usually sold to a downstream moulded parts manufacturer such as GUME for further processing into moulded parts.
HNBR is then typically processed into moulded parts using conventional methods such as extrusion, compression moulding, transfer moulding and injection moulding.
4. possibilities and limitations of HNBR
What are the advantages of HNBR?
HNBR has a number of strengths in terms of chemical, mechanical and physical properties.
HNBR is particularly characterised by its high performance in a wide temperature range from -30 to 150 °C. Furthermore, HNBR has a high chemical resistance to oils, greases, lubricants, fuels and technical media as well as to ozone and heat. The combination of these properties with high mechanical strength under tear and abrasion loads makes HNBR particularly suitable for seals, hoses and belts.
In addition, HNBR is cheaper than fluoroelastomers and yet performs better than the base polymer NBR.
HNBR can be customised for specific applications and properties thanks to its variety of compounds and the control of polymer chain growth. Special HNBR formulations with FDA conformity in accordance with 21 CFR 177.2600 for food contact and suitability for applications with gas installations in accordance with EN 549 and for drinking water applications in accordance with KTW-BWGL, W270 or comparable approvals are available. Carbon black or conductive fillers can also be used to transform the electrical conductivity of the originally well-insulating material into an electrically conductive or dissipative material. Furthermore, HNBR compounds that fulfil UL94 classifications or comparable flame retardant standards can be produced by adding halogenated polymers.
What are the disadvantages of HNBR?
The disadvantages of HNBR are particularly evident in its flexibility at low temperatures. Compared to vinyl-methyl-silicone rubber (VMQ), low-temperature optimised ethylene-propylene-diene rubber (EPDM) or acrylate-ethylene rubber (AEM), its use in very cold environments is limited and other polymers are more suitable.
5. material comparison - HNBR vs. other elastomers
The most important alternatives to HNBR in similar applications are NBR, EPDM, FKM and VMQ.
| International abbreviation | HNBR | NBR | EPDM | FKM | VMQ | |
| Hardness range (in Shore) | 50A-95A | 20A-75D | 20A-95A | 50A-90A | 20A-90A | |
| Mechanical properties at room temp. | Tear resistance | 4 | 3 | 3 | 2 | 1 |
| Elongation at break | 3 | 3 | 3 | 2 | 4 | |
| Rebound Elasticity | 2 | 2 | 3 | 0 | 3 | |
| Continue tearing resistance | 1 | 2 | 3 | 1 | 1 | |
| Abrasion resistance | 1 | 2 | 1 | 2 | 3 | |
| Compression set rest | at max. continuous operating temperature | 2 | 1 | 0 | 0 | 0 |
| at room temperature | 1 | 0 | 0 | 1 | 0 | |
| Thermal behaviour | Cooling behaviour (Tg) up to °C | -30 | -30 | -50 | -30 | -50 |
| Max. Continuous operating temperature up to °C | 150 | 110 | 130 | 220 | 220 | |
| Resistance to | Petrol | 2 | 2 | 1 | 3 | 2 |
| Mineral oil (at 100 °C) | 3 | 3 | 1 | 3 | 2 | |
| Acids (aqueous inorganic acids at RT) | 2 | 2 | 3 | 3 | 2 | |
| Alkalis (aqueous inorganic alkalis at RT) | 2 | 2 | 3 | 3 | 2 | |
| Water (at 100 °C, distilled) | 2 | 2 | 3 | 3 | 2 | |
| Weather and ozone | 2 | 2 | 3 | 3 | 3 | |
Table 1: Comparison of the properties of HNBR with other rubber and silicone materials
Compared to FKM, HNBR offers lower temperature and media resistance, but impresses with its high mechanical robustness and good resistance to oils and hot oils at significantly lower material costs. Examples of applications can be found in the HNBR seal or the HNBR O-ring, which are used when the higher performance level of FKM is not required, but there are higher requirements than with classic NBR materials. Compared to NBR, HNBR can withstand significantly longer thermal loads and HNBR has advantages over EPDM and VMQ in materials with very wide temperature windows.
Cost comparison
In a cost comparison with technical elastomers, HNBR is positioned in the upper, but not the highest, cost segment. With a cost factor of x2.9, HNBR is significantly higher than the standard elastomers EPDM and NBR (cost factor 1.0x). Compared to CR (cost factor x1.2) and TPE/TPU (cost factor x1.3), HNBR is more than twice as expensive. Silicone (LSR) with a cost factor of x1.4 and silicone (HTV) with a cost factor of x1.8 also remain below the cost of HNBR.
| Mate rial | EPDM | NBR | CR | TPE/ TPU | Silicone (LSR) | Silicone (HTV) | HNBR | FKM | FVMQ |
|---|---|---|---|---|---|---|---|---|---|
| Cost factor | x 1,0 | x 1,0 | x 1,2 | x 1,3 | x 1,4 | x 1,8 | x 2,9 | x 3,7 | x 3,6 |
Table 2: Cost comparison of HNBR with other rubber and silicone materials
The price of HNBR therefore also reflects its high-performance properties and its role as a high-performance material in technical applications. FVMQ (cost factor x3.6) is also more expensive than HNBR and is therefore one of the more expensive speciality elastomers. Only FKM (cost factor x3.7) is available as a more expensive alternative and again clearly outperforms HNBR - but is also characterised by even higher resistance to working temperatures and very good ageing resistance in hot, chemically aggressive media.
